Category: Lab
Schumann Lab – Affiliated Scientist
Host Pathogen Interaction
Projects
Lab Members
shruthi.thada(at)charite.de
+49 (0)30 450 524 234
anjana.gopinath(at)charite.de
+49 (0)30 450 524 234
Publications
Feldmeier and Ignatius Lab – Affiliated Scientists
Infection Epidemiology
Projects
- Morbidity assessment in tropical parasitic skin diseases
- Control of tungiasis in the Amazon lowlands of Colombia
- Diagnosis of scabies in resource-poor settings
- Pathogenesis of severe tick-bite-associated dermatosis, the Cauca Province, Southwest Colombia
Projects in planning
- „Tungiasis in East Africa-an interdisciplinary approach to understand the interactions between parasite and host” together with Dr. Jürgen Krücken, Institute of Parasitology and Tropical Veterinary Medicine, Freie Universität Berlin (FUB); Dr. Ulrike Fillinger, International Centre of Insect Physiology and Ecology, Kenia (ICIPE); Prof. Dr. Charles Waiswa, School of Veterinary Medicine and Animal Resources, College of Veterinary Animal Resources and Biosecurity, Makerere University, Uganda (MAK)
- “Transmission of hazardous pathogens through traditional treatment of tungiasis”, together with Prof. Dr. Felix Drexler, Institut für Virologie, Universität Bonn
Lab Members
Felix Reichert
Susanne Wiese
Publications
Gräser Lab
Molecular Mycology
yvonne.graeser(at)charite.de
Hindenburgdamm 27/Krahmerstr. 1
+49 30 450 524 066 (office)
+49 30 450 524 025
yvonne.graeser(at)charite.de
Hindenburgdamm 27/Krahmerstr. 1
+49 30 450 524 066 (office)
+49 30 450 524 025

Projects
(1) Molecular diagnostics of fungi
- Development of new molecular methods (PCR, real time PCR, microarray technique) for the detection of dermatophyte species in collaboration with Biotech companies
- Development of proficiency tests for external quality controls
(2) Epidemiological studies of selected dermatophyte species using molecular typing methods
- MLMT (Multi Locus Microsatellite Typing) – A technique to analyze the population structure, identify routes of transmission and sources of infection (by using the genome variability) of strains of Microsporum canis and Trichophyton rubrum
(3) Analysis and distribution of mating type loci (HMG box, alpha domain) of sexual and clonal reproducing dermatophyte species
Funding
- Reference center for Dermatophytes by Robert Koch Institut (RKI)
- Development of kits for the molecular diagnostics of Dermatophytes by TBI/company and AIF
Lab Members
susanne.kosanke(at)charite.de
+49 (0)30 450 524 063 (office)
+49 (0)30 450 524 025 (lab)
susanne.kosanke(at)charite.de
+49 (0)30 450 524 063 (office)
+49 (0)30 450 524 025 (lab)
christiane.kupsch(at)charite.de
+49 (0)30 450 524 063 (office)
+49 (0)30 450 524 025 (lab)
christiane.kupsch(at)charite.de
+49 (0)30 450 524 063 (office)
+49 (0)30 450 524 025 (lab)
Publications
Heimesaat and Bereswill Lab
Gastrointestinal Microbiology
stefan.bereswill(at)charite.de
+49 (0)30 450 524 328
stefan.bereswill(at)charite.de
+49 (0)30 450 524 328
markus.heimesaat(at)charite.de
+49 (0)30 450 524 318
markus.heimesaat(at)charite.de
+49 (0)30 450 524 318
Projects
Gastrointestinal Microbiology
Our group is specialized in the global analyses of microbial communities in the mammalian gastrointestinal (GI) tract. We characterize interactions of gut bacteria with host cells and investigate the microflora composition in situations of intestinal inflammation.
Campylobacter and Helicobacter research
Another research branch focussing on pathogenic bacteria in the GI tract, aims to further understanding of the biology of Campylobacter and Helicobacter species. We investigate molecular mechanisms underlying environmental adaptation, intestinal colonization capacity, and virulence.
Other projects
Evaluation of molecular strategies for prevention and therapy of intestinal Graft-versus-Host-Disease after allogeneic stem cell transplantation in mouse models (funded by the “Deutsche José Carreras Leukämie-Stiftung e. V.”).
Lab Members
nizar.shayya(at)charite.de
sebastian.schmidt(at)charite.de
+49 (0)30 450 524 274
ines.puschendorf(at)charite.de
+49 (0)30 450 524 345
gernot.reifenberger(at)charite.de
+49 (0)30 450 524 248
ulrike.fiebiger(at)charite.de
+49 30 450 524 474 (lab)
+49 30 450 524 319 (office)
ulrike.fiebiger(at)charite.de
+49 30 450 524 474 (lab)
+49 30 450 524 319 (office)
alexandra.bittroff-leben(at)charite.de
+49 30 450 524 474 (lab)
+49 30 450 524 319 (office)
alexandra.bittroff-leben(at)charite.de
+49 30 450 524 474 (lab)
+49 30 450 524 319 (office)
Publications
| 1. Fetal meconium does not have a detectable microbiota before birth. |
| Kennedy KM, Gerlach MJ, Adam T, Heimesaat MM, Rossi L, Surette MG, Sloboda DM, Braun T. |
| Nat Microbiol. 2021 Jul;6(7):865-873. doi: 10.1038/s41564-021-00904-0. Epub 2021 May 10. |
| PMID: 33972766 |
| 2. Peptidase PepP is a novel virulence factor of Campylobacter jejuni contributing to murine campylobacteriosis. |
| Heimesaat MM, Schmidt AM, Mousavi S, Escher U, Tegtmeyer N, Wessler S, Gadermaier G, Briza P, Hofreuter D, Bereswill S, Backert S. |
| Gut Microbes. 2020 Nov 9;12(1):1770017. doi: 10.1080/19490976.2020.1770017. Epub 2020 Jun 25. |
| PMID: 32584649 |
| 3. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. |
| Mousavi S, Escher U, Thunhorst E, Kittler S, Kehrenberg C, Bereswill S, Heimesaat MM. |
| Sci Rep. 2020 Feb 19;10(1):2921. doi: 10.1038/s41598-020-59890-8. |
| PMID: 32076081 |
| 4. Murine Fecal Microbiota Transplantation Alleviates Intestinal and Systemic Immune Responses in Campylobacter jejuni Infected Mice Harboring a Human Gut Microbiota. |
| Heimesaat MM, Mrazek K, Bereswill S. |
| Front Immunol. 2019 Sep 24;10:2272. doi: 10.3389/fimmu.2019.02272. eCollection 2019. |
| PMID: 31616437 |
| 5. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. |
| Robak OH, Heimesaat MM, Kruglov AA, Prepens S, Ninnemann J, Gutbier B, Reppe K, Hochrein H, Suter M, Kirschning CJ, Marathe V, Buer J, Hornef MW, Schnare M, Schneider P, Witzenrath M, Bereswill S, Steinhoff U, Suttorp N, Sander LE, Chaput C, Opitz B. |
| J Clin Invest. 2018 Aug 1;128(8):3535-3545. doi: 10.1172/JCI97065. Epub 2018 Jul 16. |
| PMID: 29771684 |
| 6. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. |
| Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P, Scheffold A, Kühl AA, Bereswill S, Heimesaat MM. |
| Front Immunol. 2017 Apr 19;8:397. doi: 10.3389/fimmu.2017.00397. eCollection 2017. |
| PMID: 28469619 |
| 7. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. |
| Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, Vogt K, Kollet J, Babel N, Sawitzki B, Schwarz C, Bereswill S, Heimesaat MM, Heine G, Gadermaier G, Asam C, Assenmacher M, Kniemeyer O, Brakhage AA, Ferreira F, Wallner M, Worm M, Scheffold A. |
| Cell. 2016 Nov 3;167(4):1067-1078.e16. doi: 10.1016/j.cell.2016.09.050. Epub 2016 Oct 20. |
| PMID: 27773482 |
| 8. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. |
| Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. |
| Cell Rep. 2016 May 31;15(9):1945-56. doi: 10.1016/j.celrep.2016.04.074. Epub 2016 May 19. |
| PMID: 27210745 |
| 9. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. |
| Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. |
| Stroke. 2016 May;47(5):1354-63. doi: 10.1161/STROKEAHA.115.011800. Epub 2016 Apr 7. |
| PMID: 27056982 |
| 10. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. |
| Muñoz M, Eidenschenk C, Ota N, Wong K, Lohmann U, Kühl AA, Wang X, Manzanillo P, Li Y, Rutz S, Zheng Y, Diehl L, Kayagaki N, van Lookeren-Campagne M, Liesenfeld O, Heimesaat MM, Ouyang W. |
| Immunity. 2015 Feb 17;42(2):321-331. doi: 10.1016/j.immuni.2015.01.011. Epub 2015 Feb 10. |
| PMID: 25680273 |
Development and function of the innate immune system
Current Research
The Diefenbach laboratory studies development and function of the innate immune system, in particular of innate lymphoid cells (ILC). A current focus is to obtain a molecular understanding of how the innate immune system, by integrating environmental signals, contributes to tissue physiology. Recent studies have revealed ever more intriguing relationships between innate immune system components and basic developmental and biologic processes that are likely to reveal unsuspected pathways by which the immune system might be plumbed to improve health and healthspan.

PROJECTS
Transcriptional and epigenetic control of ILC development and function
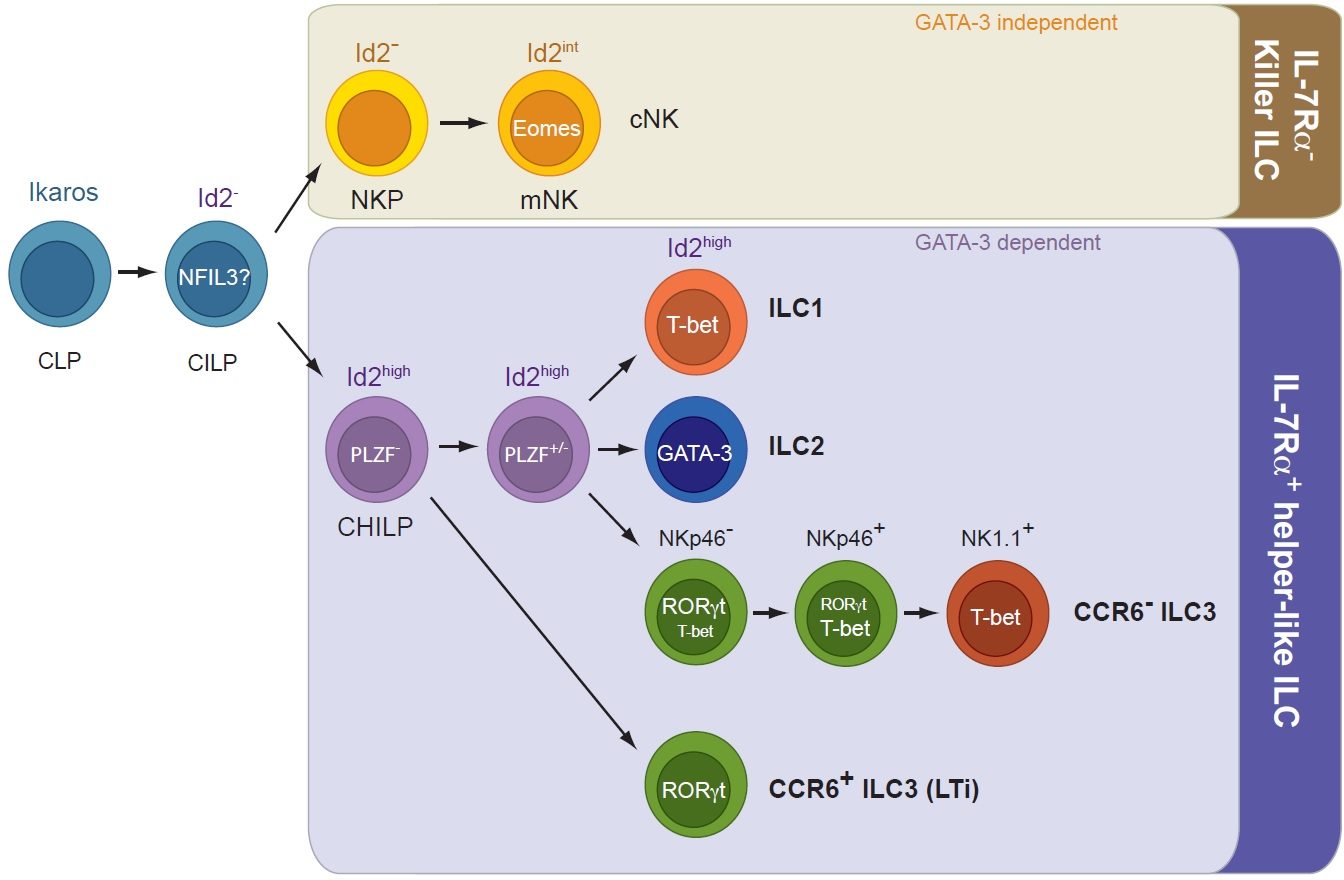
Our lab has recently contributed to the discovery of innate lymphoid cells (ILCs), a group of tissue-resident innate lymphocytes located at border surfaces that release cytokines specifically acting on epithelial cells thereby contributing to tissue homeostasis and to the adaptation of the host in response to noxious compounds and tissue damage. A focus has been the analysis of transcriptional programs controlling lineage specification, commitment and function of ILCs. We recently identified a common, Id2-expressing progenitor to all ‘helper-like’ ILC lineages, the CHILP. Interestingly, the CHILP differentiated into ILC2 and ILC3 lineages but not into conventional natural killer (cNK) cells. Instead, the CHILP gave rise to a peculiar NKp46+ IL-7Ra+ ILC population that required the transcription factor T-bet for specification and was distinct of cNK cells or other ILC lineages, the enigmatic ILC1. Using genetics and single cell-seq methodology, we investigate the transcriptional and epigenetic programs that underlie the early commitment to the ILC lineage and the signaling machinery driving ILC fate decisions.
Publications
Hernandez, P., T.Mahlakoiv, I.Yang, V.Schwierzeck, N.Nguyen, F.Guendel, K.Gronke, B.Ryffel, C.Hoelscher, L.Dumoutier, J.C. Renauld, S.Suerbaum, P.Staeheli, A.Diefenbach. 2015. Interferon-λ and interleukin-22 cooperate for the induction of interferon-stimulated genes and control of rotavirus infection. Nature Immunology. 16:698-707.
Klose, C.S.N., M.Flach, L.Möhle, L.Rogell, T.Hoyler, C.Fabiunke, K.Ebert, D.Pfeifer, V.Sexl, D.Fonseca Pereira, R.G.Domingues, H.Veiga-Fernandes, S.Arnold, I.R.Dunay, Y.Tanriver, and A.Diefenbach. 2014. Differentiation of type 1 ILCs from a common progenitor to helper-like innate lymhoid cell lineages. Cell. 157:340-356.
Diefenbach, A., Colonna, M., and Koyasu, S. 2014. Development, differentiation and diversity of innate lymphoid cells. Immunity. 41:354-365.
Klose, C.S.N., E.A.Kiss, V.Schwierzeck, K.Ebert, T.Hoyler, Y.d’Hargues, N.Göppert, A.L.Croxford, A.Waisman, Y.Tanriver, and A.Diefenbach. 2013. A T-bet gradient controls the fate and function of CCR6– RORγt+ innate lymphoid cells. Nature. 494:261-265.
Hoyler, T., C.S.N.Klose, A.Souabni, A.Turqueti-Neves, D.Pfeifer, E.L.Rawlins, D.Voehringer, M.Busslinger, and A.Diefenbach. 2012. The transcription factor GATA3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 37:634-648.
Vonarbourg, C., A.Mortha, V.L.Bui, P.Hernandez, E.A.Kiss, T.Hoyler, M.Flach, B.Bengsch, R.Thimme, C.Hölscher, M.Hönig, U.Pannicke, K.Schwarz, C.F.Ware, D.Finke, and A.Diefenbach. 2010. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity. 33:736-751.
Sanos, S.L., V.L.Bui, A.Mortha, K.Oberle, C.Heners, C.Johner, and A.Diefenbach. 2009. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nature Immunology. 10:83-91.
The role of the innate immune system in the adaptation of multicellular organisms to their environments
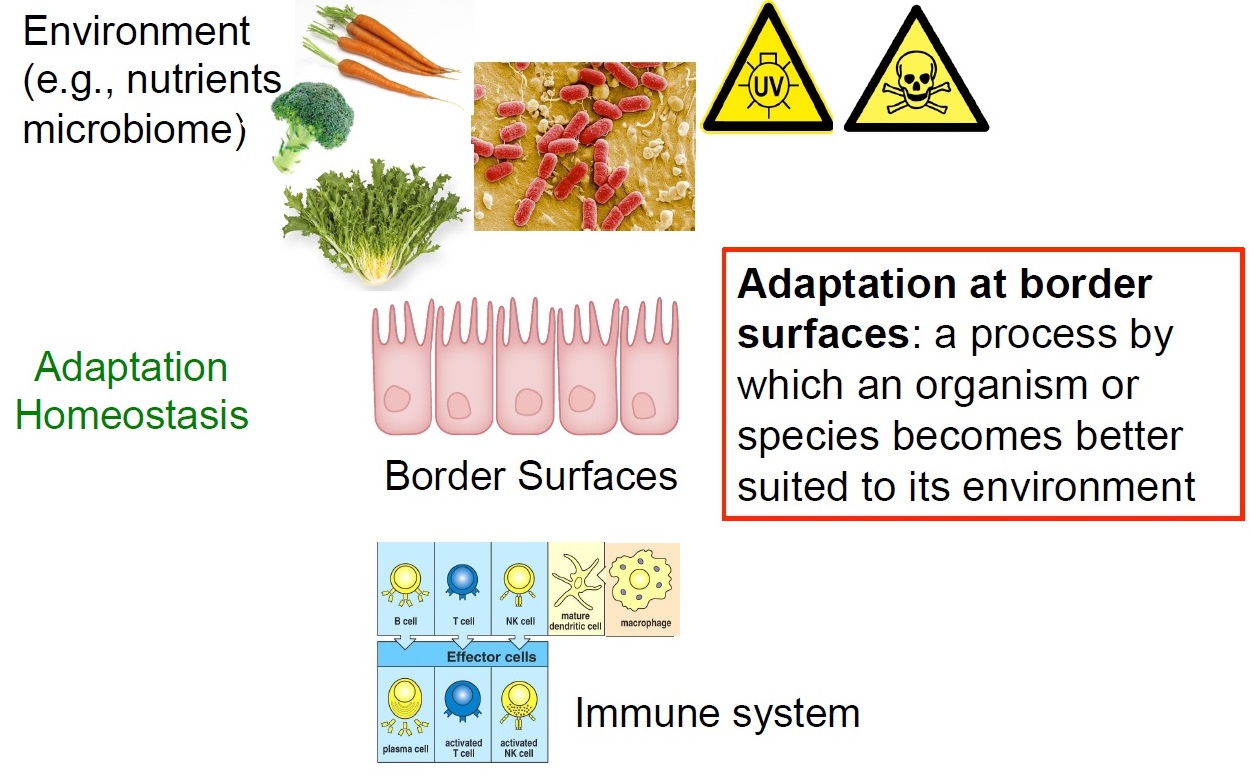
ILC are tissue-resident cells with minimal replenishment from circulating cells. By virtue of their tissue-residency, ILC are involved in processes that are not conventionally linked to the immune system such as tissue repair and growth, differentiation of epithelial cells, morphogenesis and organ homeostasis. The role of ILC as sensors for various infractions to homeostasis and their ever more intriguing roles in regulating metabolism, epithelial differentiation, and epithelial integrity are a focus of our current research. We propose that ILC are an important hub in various homeostatic control circuits thereby contributing to successful adaptation to our habitats. It is believed that ILC are important components in the maintenance of epithelial homeostasis, epithelial repair and in fortifying the epithelial barrier against infractions of its integrity. This has been best documented for the intestinal epithelial barrier, where beneath the mucus, a single layer of epithelial cells constitutes the only border between the outer intestinal environment and the body’s inner linings. Such homeostatic networks have been selected in millions of years of co-evolution with the environment and may be be plumbed to improve health and healthspan.
We have recently identified one particular homeostatic circuit that controls the response of epithelial stem cells to DNA damage. ILC3 and other lymphoid cells release large amounts of IL-22, a cytokine exclusively acting on non-hematopoietic cells. IL-22 directly regulated components of the DNA damage response (DDR) signaling network in intestinal epithelial stem cells. Intestinal stem cells deprived of IL-22 signaling had a weaker DDR in response to genotoxic stress and accumulated more tumor-promoting mutations. Interestingly, the amount of available IL-22 was regulated by genotoxic dietary components. Such glucosinolates contained in cruciferous vegetables are sensed by the aryl hydrocarbon receptor (AhR), a sensor-transcription factor in ILC3 that regulates IL-22 production. Collectively, we identified a homeostatic network protecting stem cells against perils to their genome integrity by AhR-mediated “sensing” of genotoxic components contained in diets. AhR signaling in turn ensures on-demand production of IL-22 by innate lymphocytes directly regulating components of the DDR in epithelial stem cells. Our data are among the first describing homeostatic control circuits involving components of the innate immune system that allow multicellular organisms to adapt to the intake of nutrients that have genotoxic activity.
Publications
Gronke, K., P.P.Hernández, J.Zimmermann, C.S.N.Klose, M.Kofoed-Branzk, F.Guendel, M.Witkowski, C.Tizian, L.Amann, F.Schumacher, H.Glatt, A.Triantafyllopoulou, and A.Diefenbach. 2019. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 566:249-253.
Branzk, N., K.Gronke, and A.Diefenbach. 2018. Innate lymphoid cells, mediators of tissue homeostasis, adaptation and disease tolerance. Immunol Rev. 286:86-101.
Kiss, E.A., C.Vonarbourg, S.Kopfmann, E.Hobeika, D.Finke, C.Esser, and A.Diefenbach. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 334:1561-1565.
The role of the indigenous microbiota in calibrating innate immunity
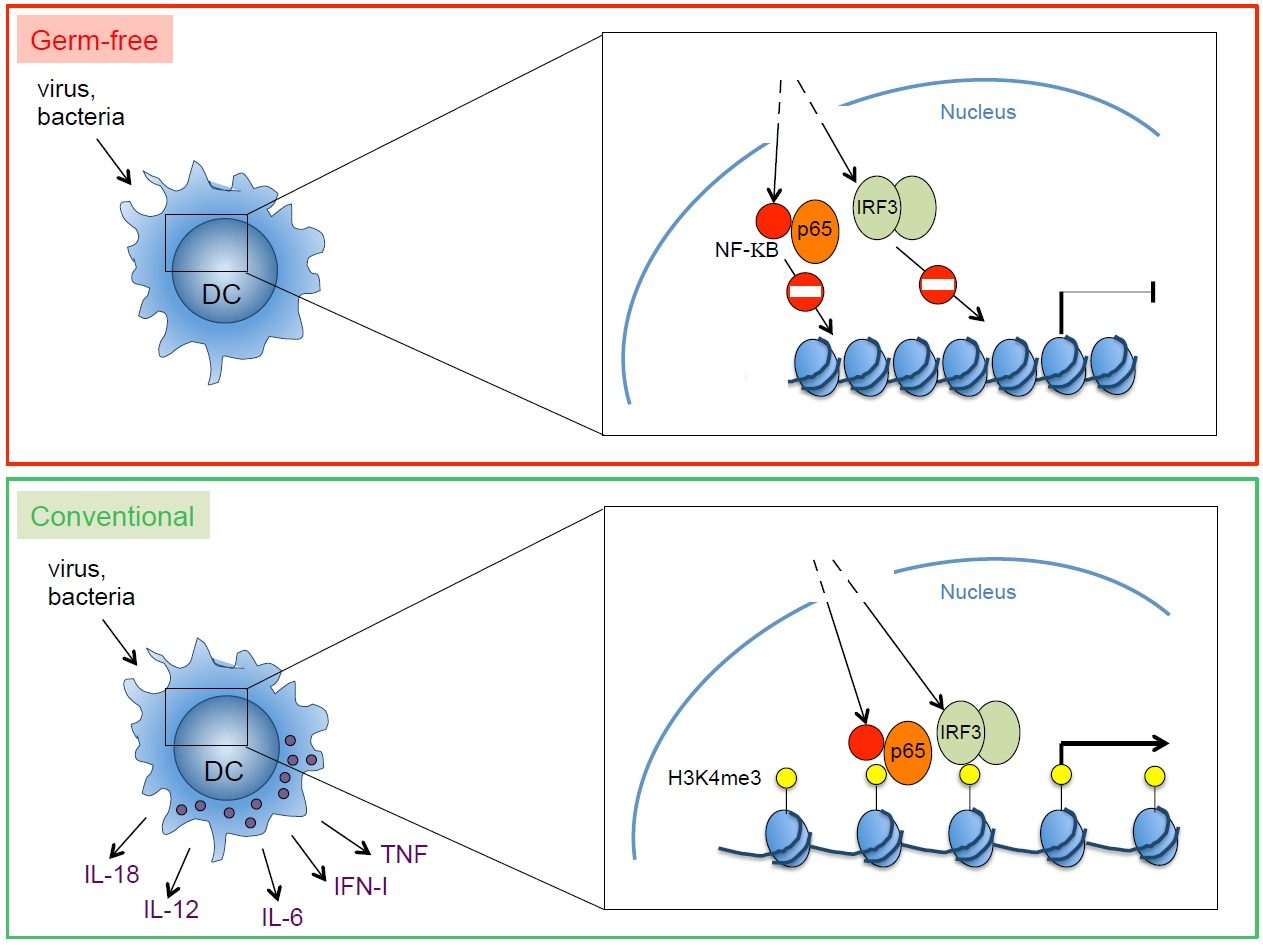
The role of environmental factors (microbiota, nutrients) for the formation of an effective and well calibrated innate immune response at barrier surfaces is recognized. Our recent work has identified an important role of the microbiota for the functional calibration of mononuclear phagocytes throughout the body. Interestingly, mononuclear phagocytes from germ-free mice were unable to produce a broad array of inflammatory cytokines in response to pathogens. Therefore, germ-free mice were unable to control viral infections. Such inability to produce cytokines reflected the failure of transcription factors activated by after pathogen recognitiom (e.g., NF-kB, IRF3) to gain access to the promoter and enhancer regions of the relevant genes. These data revealed an important and previously unrecognized role of the microbiota in poising proinflammatory gene expression by introducing epigenetic changes required for the punctual expression of cytokines needed for the protection against pathogens. Poising of gene expression within mononuclear phagocytes is likely to commence with colonization of newborns by an increasingly complex microbiota. It is intriguing to speculate that colonization of mucosal surfaces controls the development of a myeloid functional program designed to manage postnatal exposure to microbes and pathogens. During fetal development, the main function of macrophages is the removal of apoptotic cells generated in developmental processes. Expression of inflammatory genes by fetal mononuclear phagocytes may not be desirable. However, after birth, when mucosal surfaces become entry ports for pathogens, inflammatory genes are necessary to coordinate powerful and effective antimicrobial immunity. The commensal microbiota induces genome-wide changes, likely at the level of chromatin. This leads to poising of inflammatory gene expression, thereby calibrating mononuclear phagocytes at sterile, non-mucosal sites to respond promptly to pathogens.
Publications
Ganal, S.C., S.L.Sanos, C.Kallfass, K.Oberle, C.Johner, C.Kirschning, S.Lienenklaus, S.Weiss, P.Staeheli, P.Aichele, and A.Diefenbach. 2012. Priming of natural killer cells by non-mucosal mononuclear phagocytes requires instructive signals from the commensal microbiota. Immunity. 37:171-186.
Lucas, M., W.Schachterle, K.Oberle, P.Aichele, and A.Diefenbach. 2007. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 26:503-517.
Funding
LAB MEMBERS
anita.kowalczyk(at)charite.de
diego.perez-vazquez(at)charite.de
+49 30 450 524039
atsuyo.ikeda(at)charite.de
+49 30 450 524038
thordis.hohnstein(at)charite.de
+49 (0)30 450 524 049
+49 (0)30 450 524 037
bruno.rocha(at)charite.de
+49 (0)30 450 524 037
caroline.tizian(at)charite.de
+49 (0)30 450 524 037
omer.shomrat(at)charite.de
+49 (0)30 450 524 047
mario.witkowski(at)charite.de
+49 (0)30 450 524 037
michael.kofoed-branzk(at)charite.de
+49 (0)30 450 524 038
stylianos.gnafakis(at)charite.de
+49 (0)30 450 524 038
irene.mattiola(at)charite.de
+49 (0)30 450 524 038
andreas.diefenbach(at)charite.de
+49 (0)30 450 524 171





















