Neumann Lab
Lymphocyte Biology and Microenvironment
Projects
Tissue Adaptation Of Innate And Adaptive Lymphoid Cells
Chronically stimulated surfaces of the body, in particular the gastrointestinal tract (GI), are major sites where immune cells traffic and reside. Because mucosal surfaces are constantly challenged by fluctuating environmental perturbations, immune cells at these sites display a remarkable adaptive capacity in order to fend off microbial challenges and safeguard organ homeostasis. Tissue-resident innate lymphoid cells (ILC) and adaptive T helper cells are key players in protecting mucosal surfaces and maintaining immune homeostasis. To fulfil this delicate job, is has become clear that mucosal lymphocytes differ significantly in phenotype and function from their circulating or lymphoid-organ counterparts.
The Neumann lab is interested in the molecular basis of lymphocyte adaption to mucosal organs. The goal of our research is to understand the genetic, epigenetic and transcriptional mechanisms that determine the tissue-specific functions of distinct lymphocyte populations. Furthermore, we aim to identity the specific (micro)environmental cues that trigger tissue adaptation and how they influence each particular step of lymphocyte differentiation and lineage commitment.
Host – Microbiota Interactions In Health And Disease
The GI tract constitutes the largest interface between the host and the environment. Inhabited by a plethora of microorganisms (termed microbiota), the GI tract is enriched for immune cells that engage in a complex dialogue with the microbiota to maintain a state of homeostasis that is mutually beneficial. On one side, microbiota and products thereof are indispensable for shaping the development and function of the host immune system, thereby exerting multifaceted impacts on gut and systemic health. On the other side, intestinal immune cells control the composition of microbial communities, promoting colonization with mutualistic microbes and neutralization of invasive pathogens. Importantly, any imbalance of this physiological equilibrium precipitates a pathological state known as gut dysbiosis that has been linked to a variety of intestinal but also extraintestinal diseases, such as inflammatory bowel diseases, autoimmunity and metabolic disorders.
Therefore, we believe that deciphering the molecular underpinnings of the intimate cross talk between host and microbiota during homeostasis and dysbiosis may hold the key to understand many idiopathic diseases. Our research is focused on gut-resident innate and adaptive lymphocytes that specifically control host-microbiota symbiosis. Vice versa, we are interested in the ability of certain microbes to shape and heighten tissue immunity. In addition, a major focus of our research lies on the role of intestinal epithelial cells as the primary interface between host tissue and microbiota that facilitates the bidirectional communication between immune system and microbiota.
Twitter Account: https://twitter.com/lab_neumann

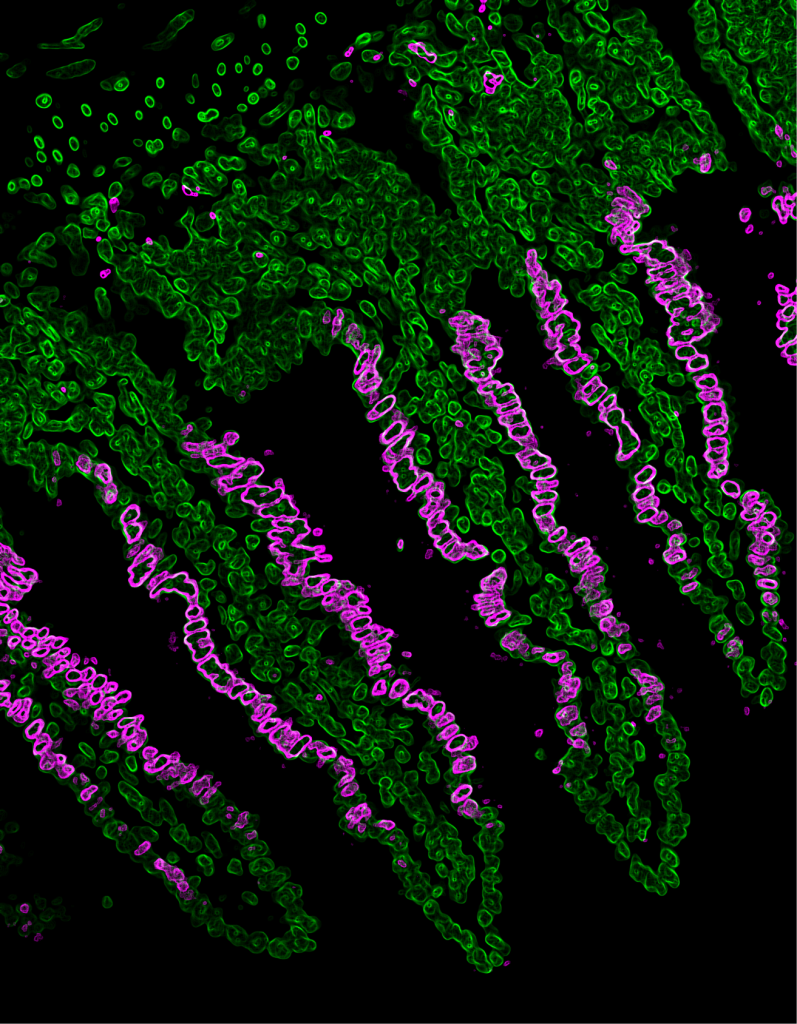
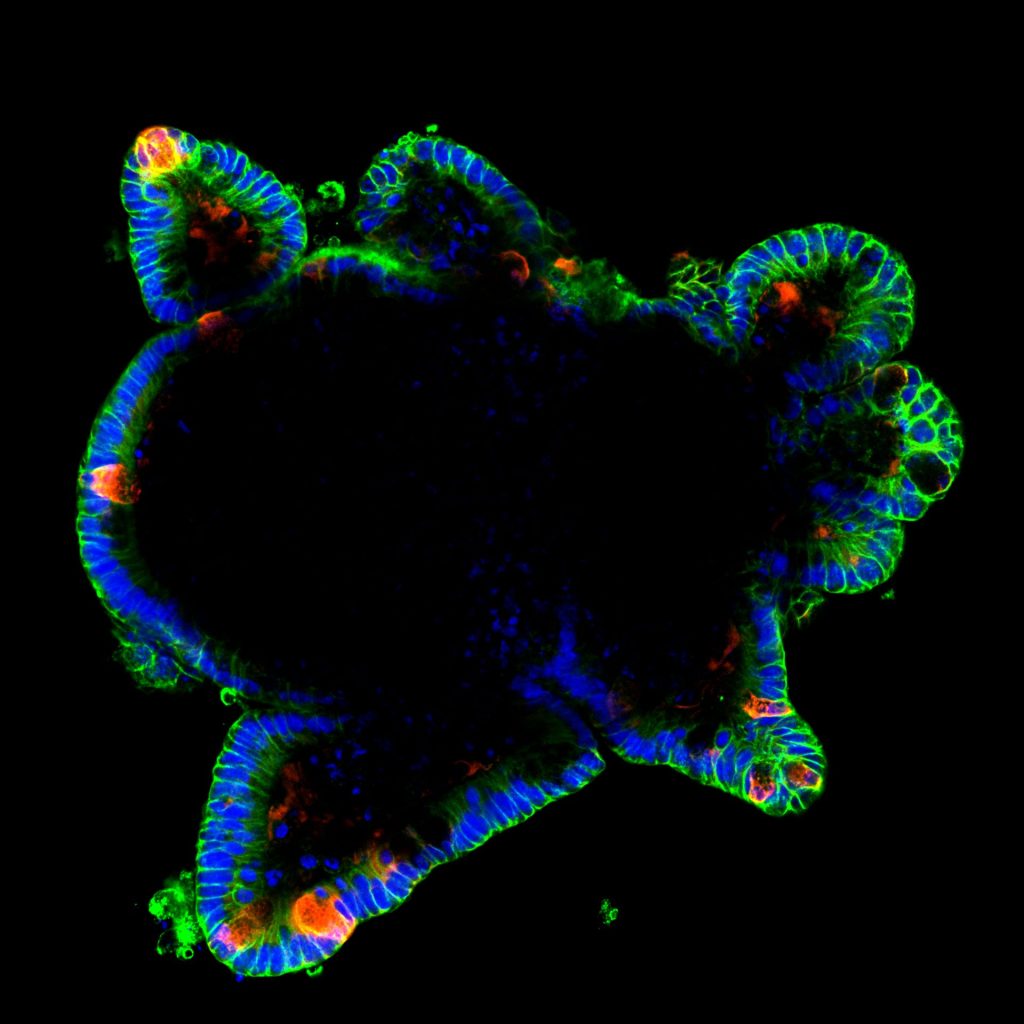
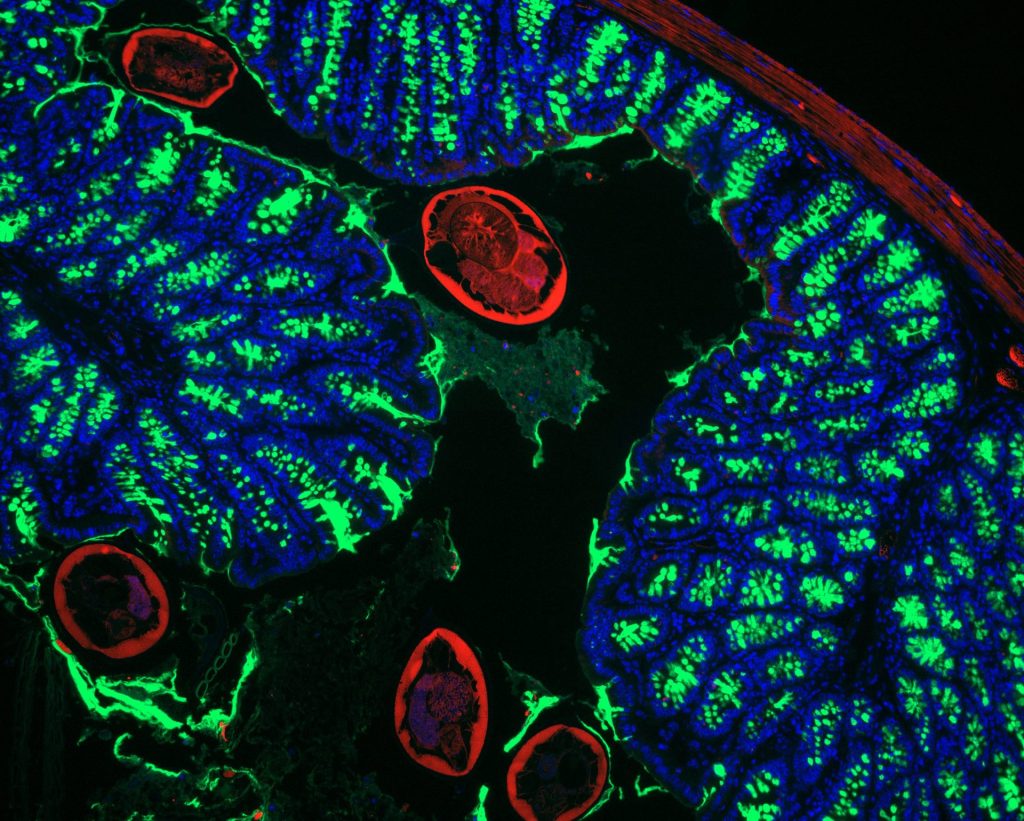
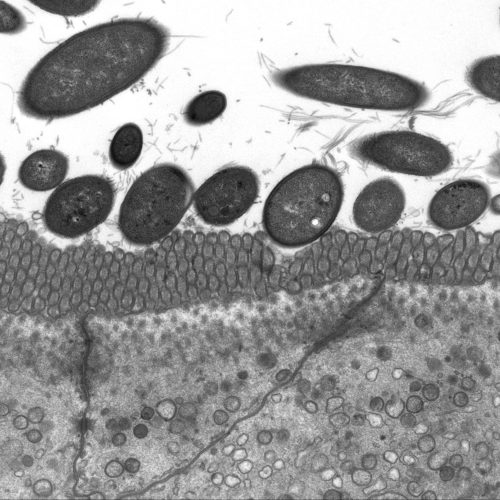
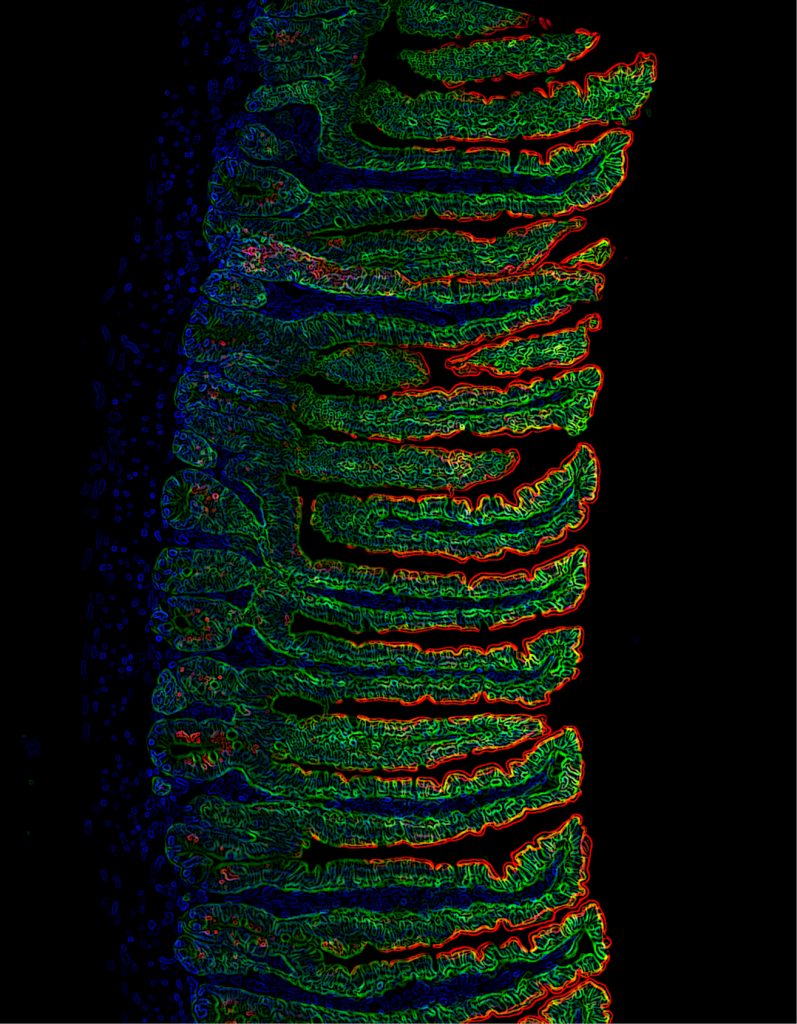
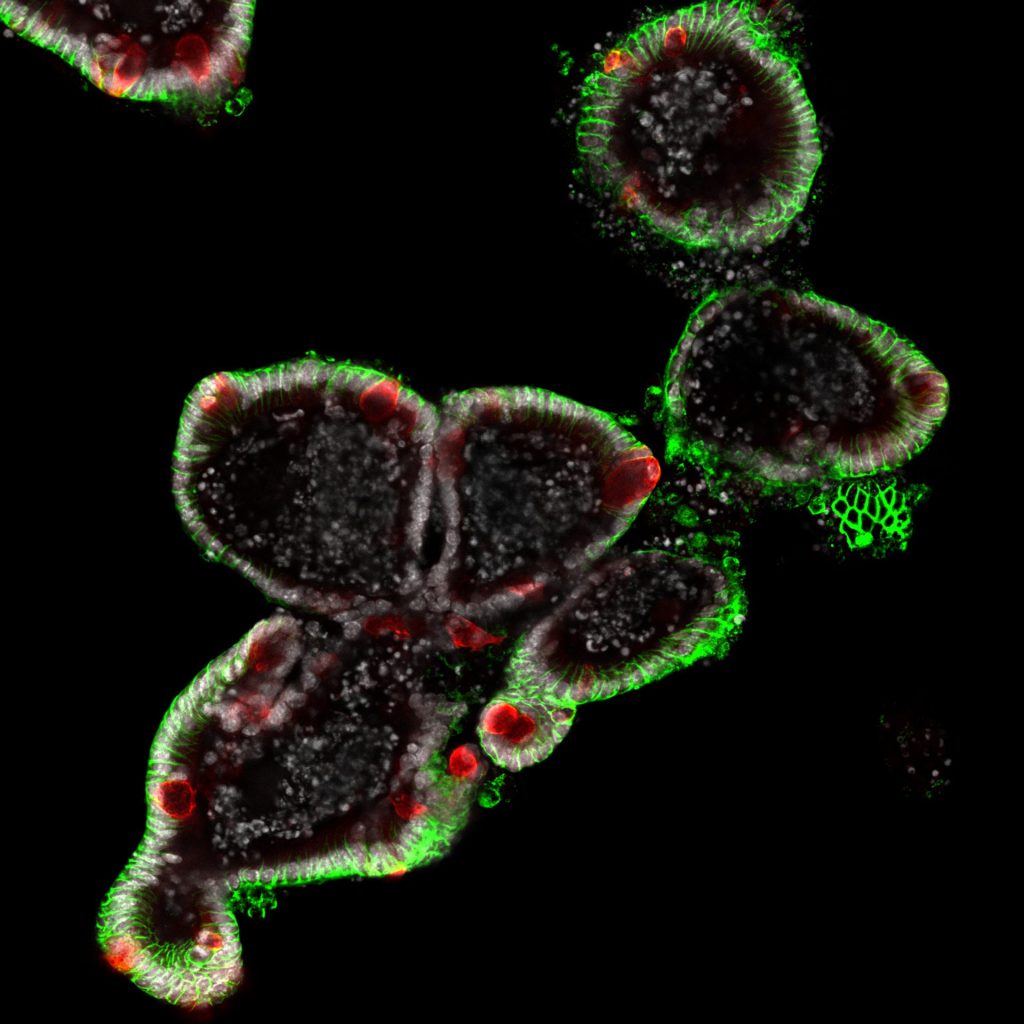

Recent Findings
Molecular regulation of enterocyte differentiation and intestinal nutrient uptake
Throughout the last years it has become increasingly clear that the intestinal epithelium has a remarkable capacity to dynamically adapt to environmental changes, such as alterations of the microbiota, in response to inflammation, injury or variations in energy supply and demand. This plasticity, which has been coined epithelial remodelling, is characterized by a coordinated reshaping of the intestinal epithelial structure and function to appropriately respond to changing environmental input and to eventually restore homeostasis. Especially, the critical importance of intestinal plasticity for maintaining energy balance of the whole organism has been highlighted, yet the molecular mechanisms and transcriptional mediators governing enterocytes and epithelial nutritional remodelling have remained incompletely defined.
We recently identified c-Maf as a novel key transcriptional regulator of small intestinal enterocytes (Cosovanu et al., JEM 2022). c-Maf, whose expression is determined by opposing Noggin/BMP signals, critically controls the differentiation and function of enterocytes, especially their capacity to sense and take up nutrients. Importantly, the epithelial cell-specific deletion of c-Maf not only compromises the organismal metabolic and nutritional status (due to defects in intestinal nutrient uptake), but also affects the abundance of intraepithelial lymphocytes and commensal epithelial cell-attaching Segmented Filamentous Bacteria, highlighting the close interdependence between intestinal epithelial cell function, the intestinal immune system and microbiota.
Currently, we are studying the role of epithelial c-Maf in experimental models of intestinal remodelling and inflammation, in which nutrient sensing and absorption are dynamically regulated and/or pathologically altered. In addition, we are determining the translational relevance of our murine studies by assessing c-Maf expression and function in human enterocytes in health and intestinal inflammation.
Balancing the gut – How the immune system maintains a healthy gut microbiota
We recently uncovered a critical mechanism that controls immune reactions against intestinal microorganisms (Neumann et al., Nature Immunology 2019). By focussing on so-called regulatory T cells (Treg), which prevent harmless microorganisms in the intestine from being attacked by the immune system, we identified a transcription factor, c-Maf, to be critical for the development and function of specific Treg cells in the gut. Deletion of c-Maf in Treg cells resulted in uncontrolled intestinal immunity to the microbiota and a profound microbial dysbiosis. The change in microbiota composition proved remarkably stable. When we transferred the altered microbiota to mice with intact c-Maf-dependent Treg cells, we similarly observed an overreaction of the intestinal immune system, indicating that both the immune system and the microbiota mutually contribute to establishing and maintaining a balanced host-microbiota relationship.
In summary, these findings could explain how microbial dysbiosis can contribute to chronic inflammatory bowel diseases. The identification of c-Maf as a key factor employed by the intestinal immune system to maintain a healthy microbiota, as well as of the signals regulating its expression, represent novel intervention points to counteract microbial dysbiosis and to re-establish intestinal immune homeostasis.
This work was done in close cooperation with Dr Sascha Rutz (Genentech, San Francisco), Dr Axel Kallies (University of Melbourne and Walter and Eliza Hall Institute of Medical Research, Melbourne) and Dr Alexander Scheffold (Kiel University and University Medical Center-UKSH, Kiel)
Press release: https://www.charite.de/en/service/press_reports/artikel/detail/alles_im_gleichgewicht/
Lab Members
Publications
Chang Y, Bach L, Hasiuk M, Wen L, Elmzzahi T, Tsui C, Gutiérrez-Melo N, Steffen T, Utzschneider DT, Raj T, Jost PJ, Heink S, Cheng J, Burton OT, Zeiträg J, Alterauge D, Dahlström F, Becker JC, Kastl M, Symeonidis K, van Uelft M, Becker M, Reschke S, Krebs S, Blum H, Abdullah Z, Paeschke K, Ohnmacht C, Neumann C, Liston A, Meissner F, Korn T, Hasenauer J, Heissmeyer V, Beyer M, Kallies A, Jeker LT, Baumjohann D. TGF-β specifies TFH versus TH17 cell fates in murine CD4+ T cells through c-Maf. Sci Immunol. 2024 Mar
Cosovanu C, Resch P, Jordan S, Lehmann A, Ralser M, Farztdinov V, Spranger J, Mülleder M, Brachs S, Neumann C. Intestinal epithelial c-Maf expression determines enterocyte differentiation and nutrient uptake in mice. J Exp Med. 2022 Dec
Ahlers J, Mantei A, Lozza L, Stäber M, Heinrich F, Bacher P, Hohnstein T, Menzel L, Yüz SG, Alvarez-Simon D, Bickenbach AR, Weidinger C, Mockel-Tenbrinck N, Kühl AA, Siegmund B, Maul J, Neumann C*, Scheffold A*. A Notch/STAT3-driven Blimp-1/c-Maf-dependent molecular switch induces IL-10 expression in human CD4+ T cells and is defective in Crohn´s disease patients. Mucosal Immunol. 2022 Mar
*Equal contribution
Cosovanu C and Neumann C. The Many Functions of Foxp3+ Regulatory T Cells in the Intestine. Front. Immunol. 2020 Oct
Tizian C, Lahmann A, Hölsken O, Cosovanu C, Kofoed-Branzk M, Heinrich F, Mashreghi MF, Kruglov A, Diefenbach A, Neumann C. c-Maf restrains T-bet-driven programming of CCR6-negative group 3 innate lymphoid cells. Elife. 2020 Feb
Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol. 2019 Aug
Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, Liao Y, Heinrich F, Arenzana TL, Hackney JA, Eidenschenk C, Gálvez EJC, Stehle C, Heinz GA, Maschmeyer P, Sidwell T, Hu Y, Amsen D, Romagnani C, Chang HD, Kruglov A, Mashreghi MF, Shi W, Strowig T, Rutz S, Kallies A, Scheffold A. c-Maf-dependent Treg cell control of intestinal Th17 cells and IgA establishes host-microbiota homeostasis. Nat Immunol. 2019 Apr
Ekmekciu I, von Klitzing E, Neumann C, Bacher P, Scheffold A, Bereswill S, Heimesaat MM. Front MFecal Microbiota Transplantation, Commensal Escherichia coli and Lactobacillus johnsonii Strains Differentially Restore Intestinal and Systemic Adaptive Immune Cell Populations Following Broad-spectrum Antibiotic Treatment. Front Microbiol. 2017 Dec
Ekmekciu I, von Klitzing E, Fiebiger U, Neumann C, Bacher P, Scheffold A, Bereswill S, Heimesaat MM. The Probiotic Compound VSL#3 Modulates Mucosal, Peripheral, and Systemic Immunity Following Murine Broad-Spectrum Antibiotic Treatment. Front Cell Infect Microbiol. 2017 May
Ekmekciu I, von Klitzing E, Fiebiger U, Escher U, Neumann C, Bacher P, Scheffold A, Kühl AA, Bereswill S, Heimesaat MM. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front Immunol. 2017 Apr
Neumann K, Rudolph C, Neumann C, Janke M, Amsen D, Scheffold A. Liver sinusoidal endothelial cells induce immunosuppressive IL-10-producing Th1 cells via the Notch pathway. Eur J Immunol. 2015 Jul
Haftmann C, Stittrich AB, Zimmermann J, Fang Z, Hradilkova K, Bardua M, Westendorf K, Heinz GA, Riedel R, Siede J, Lehmann K, Weinberger EE, Zimmel D, Lauer U, Häupl T, Sieper J, Backhaus M, Neumann C, Hoffmann U, Porstner M, Chen W, Grün JR, Baumgrass R, Matz M, Löhning M, Scheffold A, Wittmann J, Chang HD, Rajewsky N, Jäck HM, Radbruch A, Mashreghi MF. miR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim. Eur J Immunol. 2015 Apr
Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, Janke M, Rudolph C, Mockel-Tenbrinck N, Kühl AA, Heimesaat MM, Esser C, Im SH, Radbruch A, Rutz S, Scheffold A. Role of Blimp-1 in programing Th effector cells into IL-10 producers. J Exp Med. 2014 Aug
Neumann C, Scheffold A. Therapeutische Manipulation entzündungsfördernder T-Zellen : Von Suppression zur Selbstkontrolle [Therapeutic manipulation of inflammation-promoting T cells: from suppression to self-control]. Z Rheumatol. 2013 Sep




